Sponsored by Sanofi
By Tiana Tilli, BScH, PharmD, RPh, ACPR
Province-wide physical distancing and mask requirements have lifted in British-Columbia, setting the stage for the return of influenza season this Fall.1 When COVID-19 mandates were in place during the 2020-2021 influenza season, no community circulation of influenza occurred.2 In contrast, the 2021-2022 influenza season had a late onset in March 2022 as mask mandates were starting to be removed.3,4 The public must return to relying on influenza immunization to protect against flu-related morbidity and mortality this year.
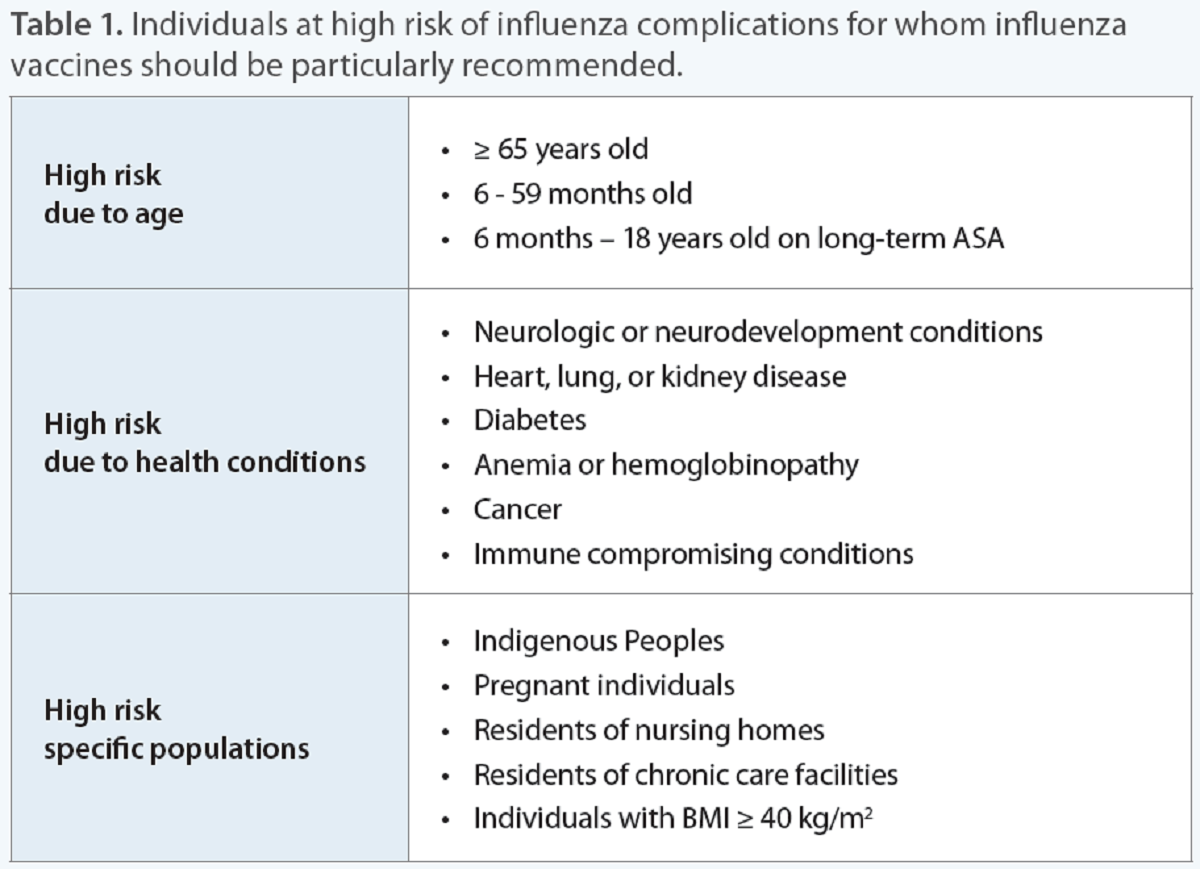
Individuals at increased risk of influenza complications
Influenza and pneumonia, together, are ranked in the top 10 leading causes of death in Canada.5 As the rates of influenza-attributed mortality increase with increased age, adults 65 years and older represent a group for which annual influenza vaccination is particularly recommended (Table 1).6,7 A systematic review found that immunizing older adults lowered the incidence of pneumonia, hospital admissions, and death.8 A yearly flu shot is recommended as the body’s immune response to influenza vaccines may diminish over time.7 Additionally, the specific influenza strains that circulate from year to year can change and so annual vaccination is needed to better match the strains expected to circulate that specific season.7
Influenza vaccines for the 2022/23 season
For the 2022/23 northern hemisphere influenza season, the strains recommended by the WHO for inclusion in the quadrivalent influenza vaccines are as below. There are differences between the strains recommendations for egg-based and cell- or recombinant-based vaccines because certain influenza viruses replicate differently in the different production systems. Different viruses, that have similar antigenic properties, are thus used to trigger similar immune responses.10
Egg Based Vaccines9:
- A/Victoria/2570/2019 (H1N1)pdm09-like virus
- A/Darwin/9/2021 (H3N2)-like virus *new this year*
- B/Austria/1359417/2021 (B/Victoria lineage)-like virus *new this year*
- B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
Cell culture- or recombinant-based vaccines9
- A/Wisconsin/588/2019 (H1N1)pdm09-like virus
- A/Darwin/6/2021 (H3N2)-like virus *new this year*
- B/Austria/1359417/2021 (B/Victoria lineage)-like virus *new this year*
- B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
Influenza vaccine technologies
Various vaccine technologies have been leveraged to develop influenza vaccines that promote strong immune responses (Table 2). Influenza vaccines are designed to trigger an immune response to the virus’ surface proteins, hemagglutinin (HA).11 HA proteins act as antigens that are recognized by the immune system and lead to antibody production.11 Improved immune response to antigen is especially important for adults 65 years and older because of their lower immune response after vaccination as a result of immunosenescence.
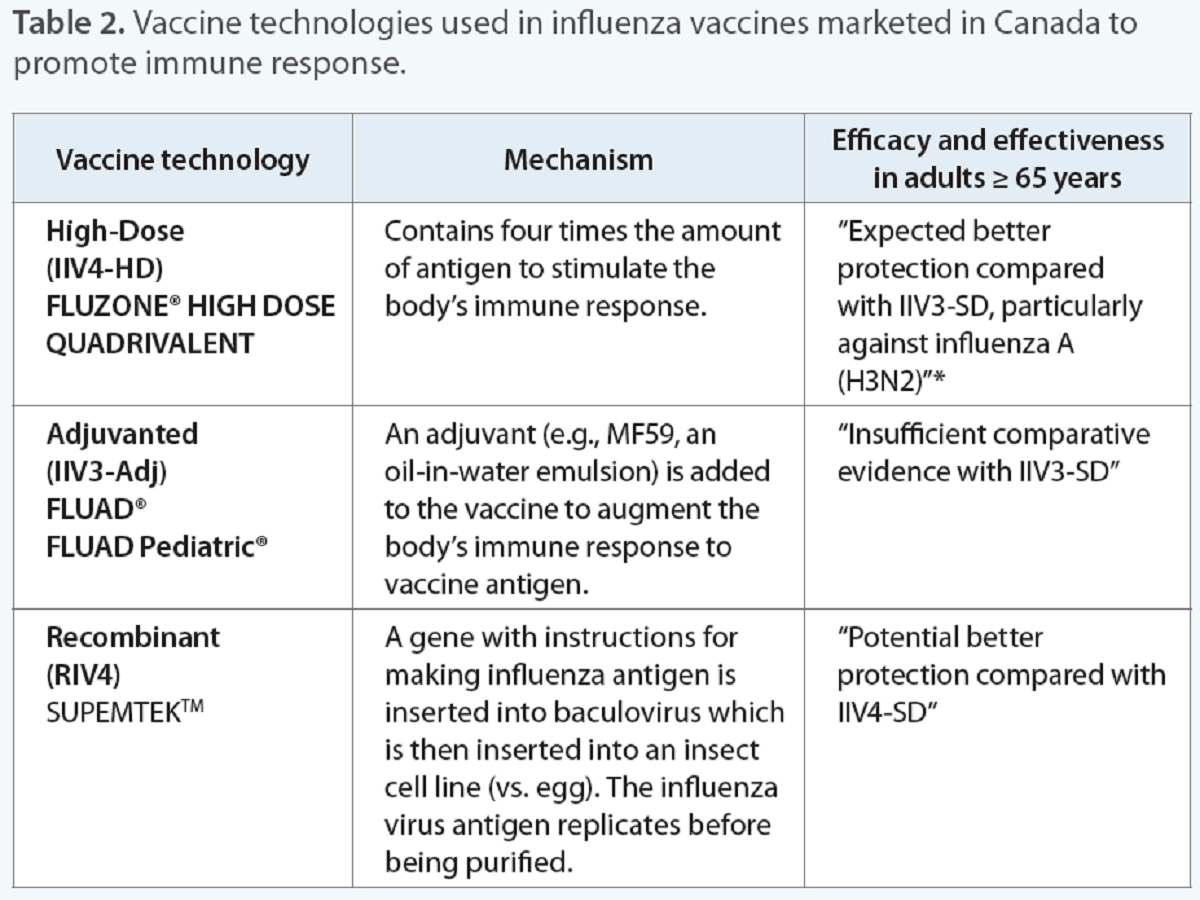
*Influenza A (H3N2) generally causes an increased burden of illness in adults ≥ 65 years old
Making recommendations to protect older adults
Since protection is achieved by two weeks after immunization, it’s best to recommend being vaccinated as early as possible in the Fall.7 Given the number of influenza vaccines available, it can be challenging to know which vaccine to recommend (Table 3). The top priority is ensuring your patient gets vaccinated. Additional considerations include the cost to the client, strains protected against and the resulting immune response.
In adults 50 years and older, the recombinant influenza vaccine was found to provide better protection compared to the standard-dose quadrivalent vaccine in a study of the 2014-2015 influenza season.12 The probability of laboratory-confirmed influenza illness-like illness was 30% lower with recombinant influenza vaccine.12
In adults 65 years and older across the United States and Canada, the high-dose influenza vaccine was 24% more effective in preventing laboratory-confirmed influenza compared to the standard-dose vaccine.13 Another study found that nursing home residents aged 65 years and older who were immunized with high-dose influenza vaccine experienced lower rates of respiratory-related hospital admissions than those vaccinated with the standard-dose vaccine.
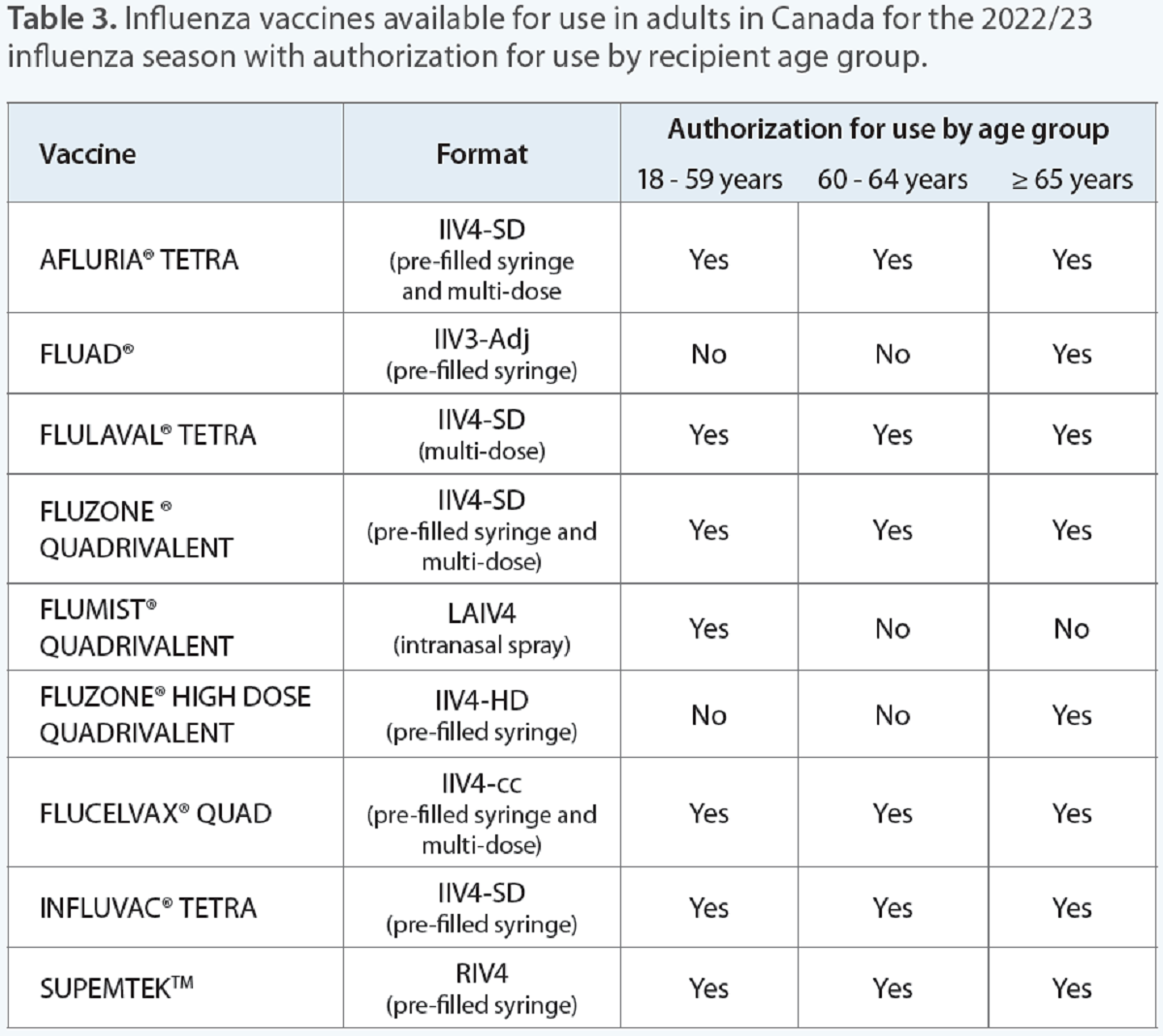
IIV4-SD: standard-dose quadrivalent inactivated influenza vaccine; IIV3-Adj: adjuvanted trivalent inactivated influenza vaccine; LAIV4; quadrivalent live attenuated influenza vaccine; IIV4-HD: high-dose quadrivalent influenza vaccine; IIV4-cc: quadrivalent mammalian cell-culture-based inactivated influenza vaccine; RIV4: quadrivalent recombinant influenza vaccine.
1. Government of British Columbia. Province-wide Restrictions. April 8, 2022. https://www2.gov.bc.ca/gov/content/covid-19/info/restrictions
2. Government of Canada. Annual Influenza Reports: FluWatch Annual Report Summary. October 27, 2021. https://www.canada.ca/en/public-health/services/diseases/flu-influenza/influenza-surveillance/annual-reports.html
3. Government of Canada. Weekly Influenza Reports: FluWatch Summary. June 24, 2022. https://www.canada.ca/en/public-health/services/diseases/flu-influenza/influenza-surveillance/weekly-influenza-reports.html
4. Government of British Columbia. B.C. Takes Next Step in Balanced Plan to Lift COVID-19 Restrictions. March 10, 2022. https://news.gov.bc.ca/releases/2022HLTH0081-000324
5. Statistics Canada. Leading Causes of Death, Total Population, By Age Group. January 24, 2022. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039401
6. Schanzer, D. L., Tam, T. W. S., Langley, J. M., & Winchester, B. T. (2007). Influenza-attributable deaths, Canada 1990–1999. Epidemiology & Infection, 135(7), 1109-1116.
7. National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2022-2023. June 8, 2022. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2022-2023.html
8. Demicheli, V., Jefferson, T., Di Pietrantonj, C., Ferroni, E., Thorning, S., Thomas, R. E., & Rivetti, A. (2018). Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews, (2).
9. World Health Organization. Recommendations Announced for Influenza Vaccine Composition for the 2022-2023 Northern Hemisphere Influenza Season. February 25, 2022. https://www.who.int/news/item/25-02-2022-recommendations-announced-for-influenza-vaccine-composition-for-the-2022-2023-northern-hemisphere-influenza-season
10. World Health Organization. Questions and Answers: Recommended Composition of Influenza Virus Vaccines for Use in the Northern Hemisphere 2022-2023 Influenza Season and Development of Candidate Vaccine Viruses for Pandemic Preparedness. February 25, 2022. https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2022-2023/202202_qanda_recommendation.pdf?sfvrsn=868a7c73_11
11. Centers for Disease Control and Prevention (CDC). Influenza (Flu): Types of Influenza Viruses. November 2, 2021. https://www.cdc.gov/flu/about/viruses/types.htm
12. Dunkle, L. M., Izikson, R., Patriarca, P., Goldenthal, K. L., Muse, D., Callahan, J., & Cox, M. M. (2017). Efficacy of recombinant influenza vaccine in adults 50 years of age or older. New England Journal of Medicine, 376(25), 2427-2436.
13. DiazGranados, C. A., Dunning, A. J., Kimmel, M., Kirby, D., Treanor, J., Collins, A., ... & Talbot, H. K. (2014). Efficacy of high-dose versus standard-dose influenza vaccine in older adults. New England Journal of Medicine, 371(7), 635-645.
14. Gravenstein, S., Davidson, H. E., Taljaard, M., Ogarek, J., Gozalo, P., Han, L., & Mor, V. (2017). Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. The Lancet Respiratory Medicine, 5(9), 738-746.
2022/2023 Publicly Funded Influenza Products
On Aug. 8, 2022, the BC Centre for Disease Control (BCCDC) released the list of publicly funded influenza products for 2022/23 flu season. As per Part 4 – Biological Products - Influenza Vaccines, the intended use of the influenza vaccines by age group are:
| Vaccine | Age Group |
| Flumist® Quadrivalent | 2 – 17 years of age* |
| Fluzone® Quadrivalent | 6 months – 64 years of age |
| Fluad® | 65 years of age and older residing in the community |
| Fluzone® High-Dose Quadrivalent | 65 years of age and older who are residents of long-term care, assisted living facilities and First Nations communities. |
*Flumist may be offered to 18-59 years of age who have needle phobia or unwilling to get another influenza vaccine.
